
Draw The Structure Of C H C H Electronic Dot Structure Chemistry My
Added Jun 9, 2014 by WebTester in Chemistry This widget gets the Lewis structure of chemical compounds. Send feedback | Visit Wolfram|Alpha Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

CHCl3 Lewis Structure, Geometry, Hybridization, and Polarity
The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Chemists usually indicate a bonding pair by a single.

42+ Ch3Cl Molecular Geometry Shape Pics GM
To determine if CH 3 Cl is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. CH 3 Cl has 4 + 3 + 7 = 14 valence electrons. Carbon goes in the middle and is bonded to three hydrogen and one chlorine atom.

CHCl3 Molecular Geometry Science Education and Tutorials
Ch3cl lewis structure explains the valence electrons that are present in the valence shell of an atom. Valence electrons are used in the bond formation to create the different molecules. Lewis structure is drawn by dots which represent the valence electrons assigned around the elements present in the molecules.
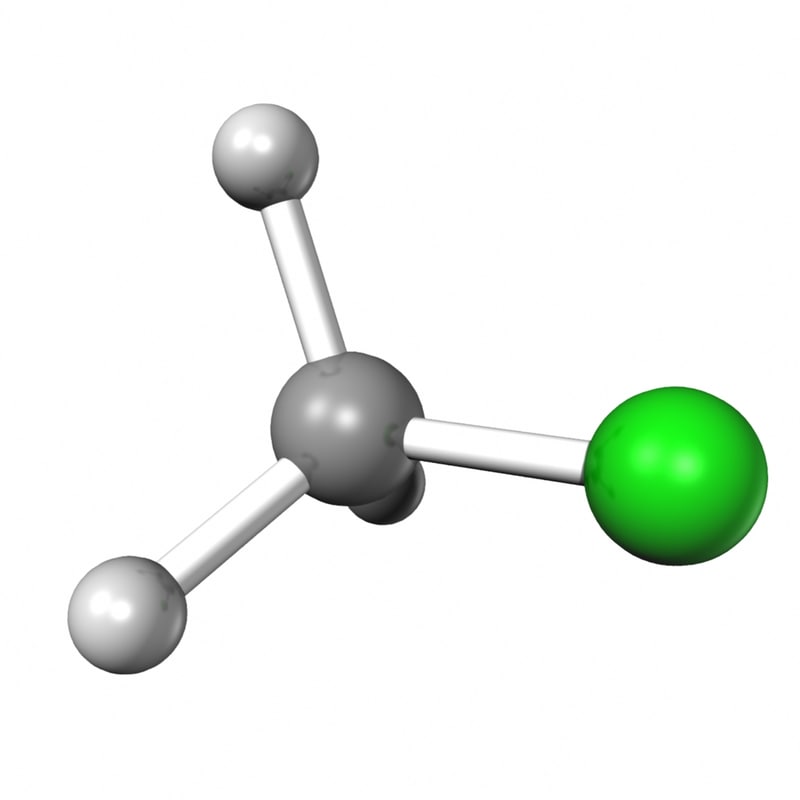
ch3cl 3d obj
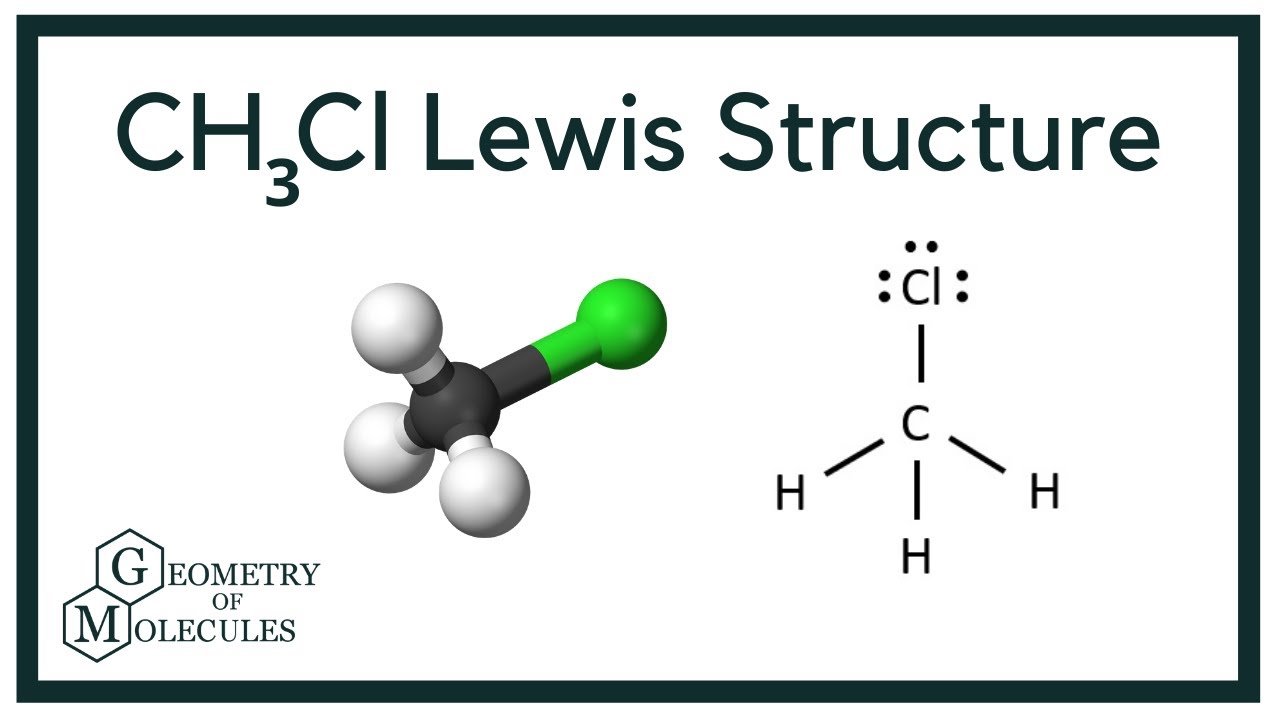
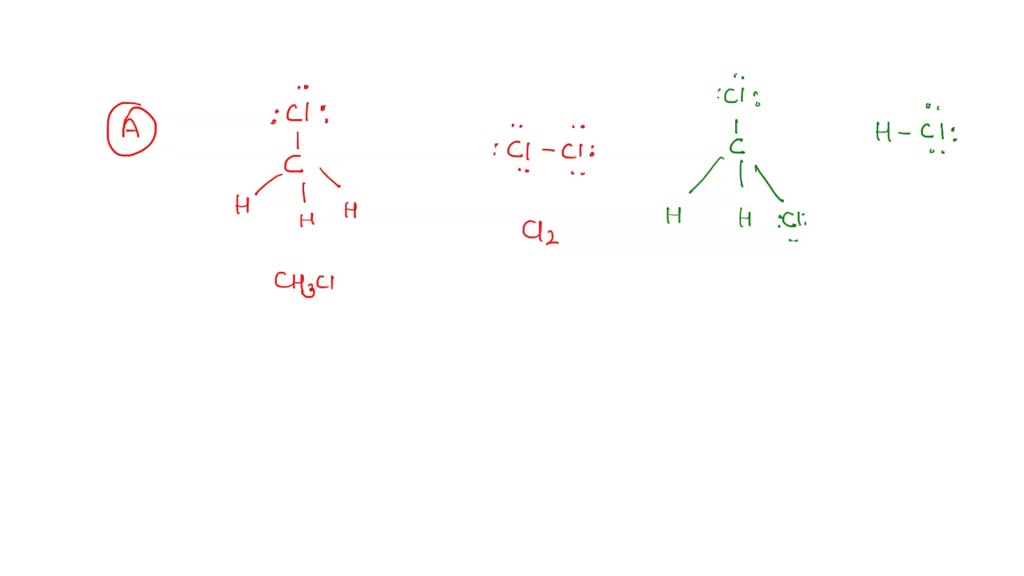
In the CH 3 Cl Lewis structure, there are four single bonds around the carbon atom, with three hydrogen atoms and one chlorine atom attached to it, and on the chlorine atom, there are three lone pairs. CH3Cl Lewis Structure - How to Draw the Lewis Structure for CH3Cl (Chloromethane) Watch on Contents Steps #1 First draw a rough sketch

So far, we’ve used 14 of the CH3Cl Lewis structure’s total 14 outermost
460 Share 99K views 10 years ago A step-by-step explanation of how to draw the CH3Cl Lewis Dot Structure (Chloromethane). For the CH3Cl structure use the periodic table to find the total.
Found Non Bonded Atoms Error sydneyfasr
The Lewis structure of CH3Cl consists of a carbon (C) atom at the center which is bonded to three hydrogens (H) atoms and one atom of chlorine (Cl). There are a total of 4 electron density regions around the central C atom in the CH3Cl Lewis structure.
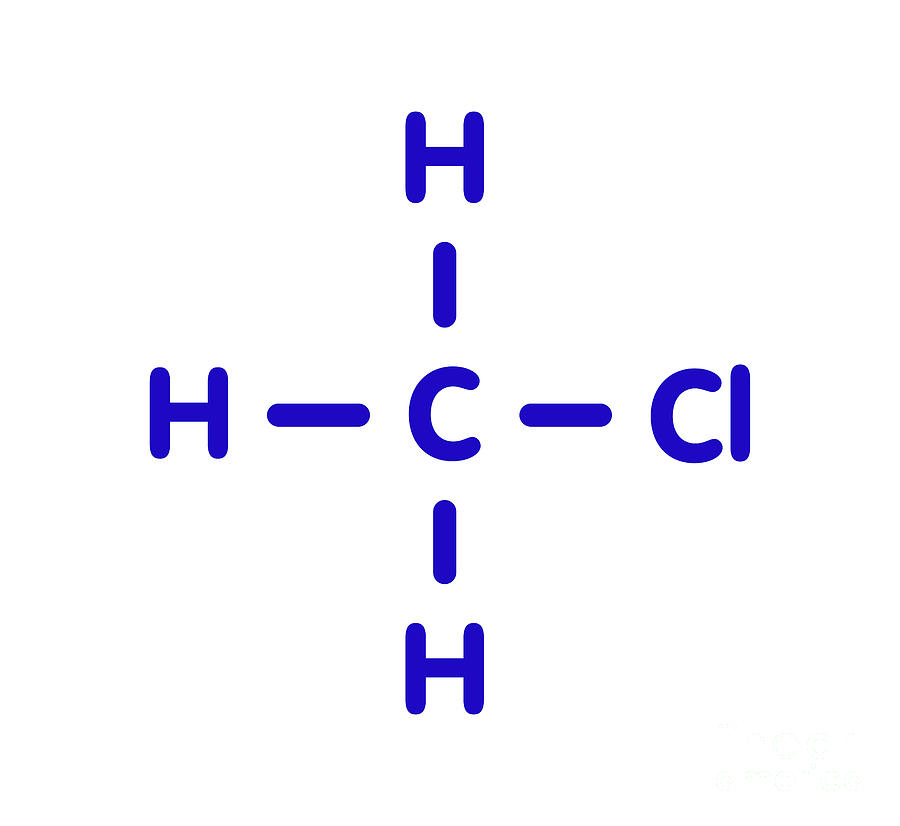
Chloromethane Molecule Photograph by Molekuul/science Photo Library
CH3Cl lewis structure has a Carbon atom (C) at the center which is surrounded by three Hydrogen atoms (H) and one Chlorine atom (Cl). There is a single bond between the Carbon (C) & Chlorine (Cl) atom as well as between the Carbon (C) and Hydrogen (H) atoms. There are 3 lone pairs on the Chlorine atom (Cl).
What Is Ch3cl Lewis Structure?
Chloromethane (CH 3 Cl) Lewis Structure Chloromethane (CH 3 Cl) contains one carbon atom, three hydrogen atoms and one chlorine atom. In the lewis structure of CH 3 Cl, carbon atom is located as the center atom and other atoms have made bonds with carbon atom. CH 3 Cl lewis structure There are three hydrogen atoms around center carbon atom.

Is CHCl3 Polar or Nonpolar? Techiescientist
Contents show Lewis Structure of Chloromethane (CH3Cl) The Lewis structure is a pictorial representation showing the electrons present in the valence shell in an atom. The diagram is drawn to determine how the valence electrons of different atoms participate in the bond formation to form a molecule.
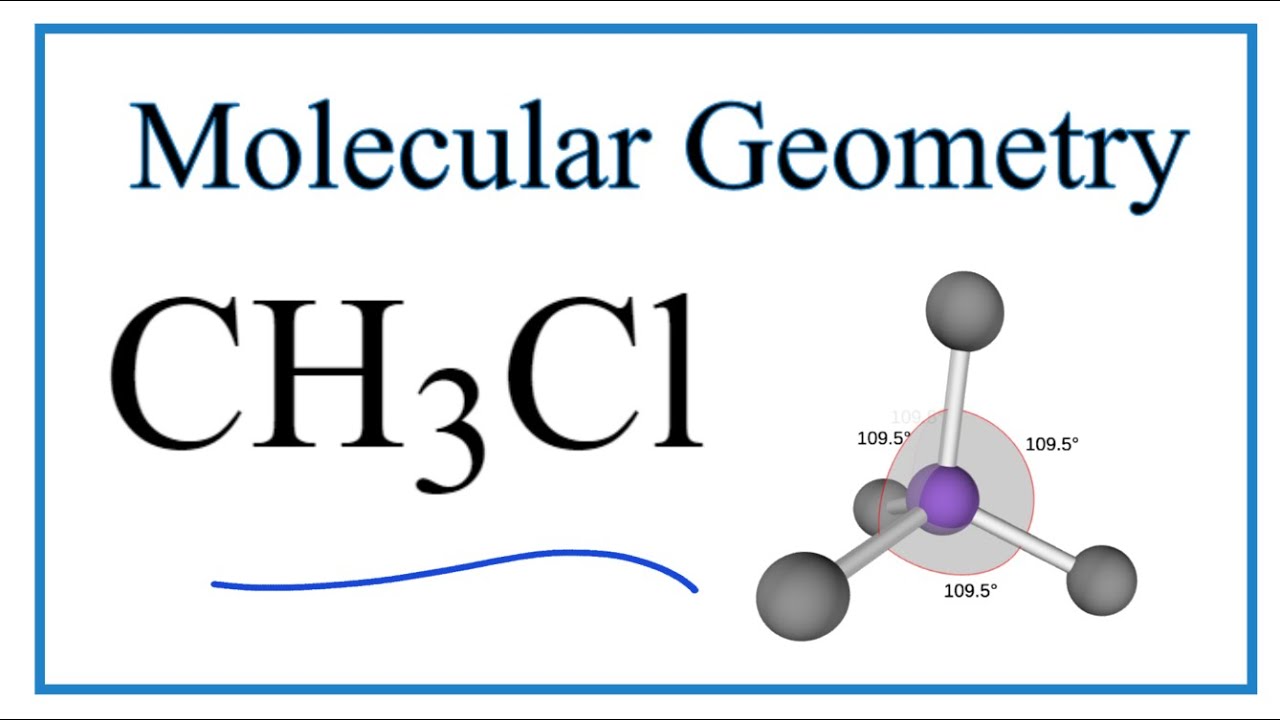
CH3Cl Molecular Geometry, Bond Angles (and Electron Geometry) YouTube
November 15, 2023 by Deep. The information on this page is fact-checked. Lewis structure of CH 3 Cl. The Lewis structure of CH3Cl contains four single bonds, with carbon in the center, and three hydrogens and chlorine on either side. There are three lone pairs on the chlorine atom, and carbon atom and hydrogen atom do not have any lone pair.

Lewis Dot Structure of CH3Cl Methyl Chloride structure
The first step is to sketch the Lewis structure of the CH3Cl molecule, to add valence electron around the carbon atom; the second step is to add valence electrons to the one chlorine and three hydrogen atoms, and the final step is to combine the step1 and step2 to get the CH3Cl Lewis Structure.
SOLVED Consider the following reaction CH3OH (l) + SOCl2 (l) > CH3Cl
Lewis structure of CH3Cl contains a single bond between the Carbon (C) & Hydrogen (H) atoms as well as between the Carbon (C) & Chlorine (Cl) atom. The Carbon atom (C) is at the center and it is surrounded by three Hydrogen (H) and one Chlorine atom (Cl). The Chlorine atom has 3 lone pairs.

Lewis Structures Made Easy Examples and Tricks for Drawing Lewis Dot
About this video -Lewis dot structure of CH3Cl, known as methyl chloride or chloromethane.Happy Reading :)

Lewis dot structure of ch3cl AnswerData
CHCl3 Lewis Structure Lewis Structure is a simple representation of how valence shell electrons are arranged in a molecule. It tells us that a bond exists between atoms but does not tell us anything about the type of bonds.

Ch3cl lewis structure, Characteristics13 Must To Know Facts Lambda Geeks
1.Determine the number of lone pairs on the terminal hydrogen atom of the CH3Cl Lewis structure. we need to calculate out how many there are on the terminal hydrogen atom of the Lewis structure. As we know clearly, the hydrogen atom has only s orbital in the ground state. It can take a maximum of two electrons.